Table of Contents
Title: Demystifying Aluminum Zirconium Tetrachlorohydrex Gly: Chemistry, Applications, and Safety Considerations
What Is Aluminum Zirconium Tetrachlorohydrex gly
It is a chemical compound that has gained significant attention in various industries due to its wide-ranging applications.
Commonly used in antiperspirants and deodorants, this compound has also found use in cosmetics, personal care products, and industrial applications.
This article will explore the chemistry, manufacturing, applications, safety regulations, and future trends related to aluminium zirconium tetrachlorohydrex gly.

Also Helful articel for you Paracetamol Side Effect, Dosage ,Uses, Mechanism of action
Chemical Properties and Composition of Aluminum Zirconium Tetrachlorohydrex Gly
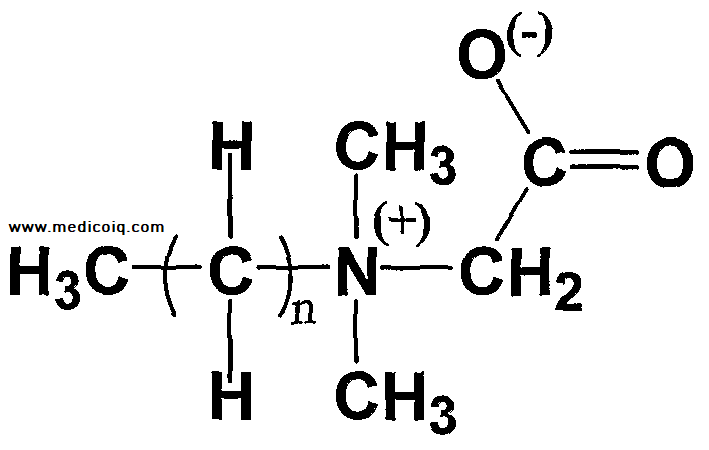
A detailed description of the chemical structure and formula –
It is a complex compound with the chemical formula [Al(OH) x ZrCl(4-x)] y , where x and y are variables depending on the specific formulation.
Physical properties, such as colour, odour, and solubility
Composition and formulation of aluminum zirconium tetrachlorohydrex gly
The composition of aluminium zirconium tetrachlorohydrex gly can vary, and it may contain other ingredients such as glycols, glycerin, and fragrances, depending on the intended use and formulation.
Synthesis and Manufacturing of Aluminum Zirconium Tetrachlorohydrex Gly
Aluminum Zirconium Tetrachlorohydrex Gly (AZG) is a commonly used antiperspirant and deodorant ingredient known for its effectiveness in controlling sweat and odor.
Understanding the synthesis and manufacturing process of AZG is essential for companies involved in the production of personal care products. Now next, we will provide an overview of the synthesis process and the raw materials used, as well as explore various methods of manufacturing aluminium zirconium tetrachlorohydrex gly.
Overview of the Synthesis Process:
The synthesis of aluminum zirconium tetrachlorohydrex gly involves several steps and requires specific raw materials. Typically, the process includes the following stages:
a. Preparation of Raw Materials: The raw materials used in the synthesis of AZG include aluminium chloride, zirconium oxychloride, glycine, and water. These materials are carefully selected for their purity and quality.
b. Mixing and Heating: The raw materials are mixed together in precise ratios and heated to specific temperatures. This step promotes the chemical reactions necessary for the formation of AZG.
c. Hydrolysis and Glycolysis: During hydrolysis, water molecules react with the aluminum chloride and zirconium oxychloride to form hydrated aluminum and zirconium species. Glycolysis involves the reaction of these hydrated species with glycine, leading to the formation of aluminum zirconium tetrachlorohydrex gly.
d. Purification and Drying: The resulting AZG solution is purified to remove impurities and unwanted by-products. The purified solution is then dried to obtain the desired solid form of aluminum zirconium tetrachlorohydrex gly.
Various Methods of Manufacturing:
Different manufacturers may employ variations in the manufacturing process to optimize the production of AZG. These variations may include:
a. Temperature and Time Control: Adjusting the temperature and reaction time can influence the yield, purity, and physical properties of AZG. Manufacturers experiment with different conditions to achieve the desired product characteristics.
b. Catalysts and Additives: Some manufacturers use catalysts or additives to enhance the synthesis process, improve the efficiency of reactions, or modify the properties of the final product. These additives can impact the stability, solubility, or sweat-controlling properties of AZG.
c. Continuous vs. Batch Production: Manufacturers may choose to employ continuous or batch production methods based on factors such as production volume, equipment capabilities, and cost-effectiveness. Each approach has its advantages and considerations.
Quality control measures during the production of Aluminum Zirconium Tetrachlorohydrex Gly (AZG) are crucial to ensure the consistency, purity, and effectiveness of the final product. Here are some key quality control measures implemented throughout the production process:
Quality Control Measures During Production
- Raw Material Inspection:
- Thoroughly inspect incoming raw materials such as aluminium chloride, zirconium oxychloride, and glycine for quality, purity, and adherence to specifications.
- Conduct rigorous testing to verify the absence of contaminants or impurities that could compromise the quality of AZG.
- Process Monitoring:
- Implement a robust process monitoring system to continuously assess critical parameters such as temperature, reaction time, pH levels, and agitation speed.
- Utilize automated sensors, data logging, and control systems to ensure consistent process conditions and adherence to predefined parameters.
- Sampling and Analysis:
- Regularly collect samples at different stages of production for comprehensive analysis and testing.
- Analyze samples using various techniques such as chromatography, spectroscopy, and microscopy to verify the chemical composition, purity, and physical characteristics of AZG.
- In-process Controls:
- Employ in-process controls to detect any deviations from the expected quality standards during the manufacturing process.
- Conduct frequent checks to verify that the intermediate products meet the desired specifications and adjust process parameters if necessary.
- Product Characterization:
- Perform thorough characterization tests on the final AZG product to evaluate its physical properties, including appearance, particle size, solubility, and moisture content.
- Conduct chemical analysis to confirm the composition and concentration of active ingredients and impurities within acceptable limits.
- Stability Testing:
- Subject AZG samples to stability testing under various conditions such as temperature, humidity, and light exposure to assess the product’s shelf-life and performance over time.
- Monitor and analyze samples periodically to ensure that AZG remains stable and maintains its intended properties throughout its designated shelf-life.
- Documentation and Record-Keeping:
- Maintain comprehensive documentation of all quality control activities, including test results, batch records, and deviations encountered during the production process.
- Establish a traceability system to track raw materials, intermediate products, and final batches, enabling effective recall management and investigation of any quality-related issues.
Know about : What Is Diclofenac Sodium Topical Gel Used For?
Mechanism Of Action, Pharmacological Action of Aluminum Zirconium Tetrachlorohydrex gly
It is a common active ingredient found in antiperspirants. It is a complex of aluminium, zirconium, chlorides, and glycine that works by blocking sweat glands in the body.
The pharmacological action of aluminum zirconium tetrachlorohydrex gly is based on its ability to form a gel-like barrier on the skin’s surface that obstructs the sweat glands and prevents excess sweat production.
The aluminium ions in this compound form a network-like structure that creates a physical barrier on the skin’s surface. This barrier helps to reduce the rate of sweat production, keeping the skin dry and reducing odour.
It also has antimicrobial properties that help to control the growth of odour-causing bacteria on the skin. The compound interacts with the proteins found in the sweat, preventing the bacteria from breaking them down into volatile compounds that cause body odour.
Use and Applications of Aluminum Zirconium Tetrachlorohydrex Gly

Safety, Regulations, and Health Concerns
The safety of AZT gly has been a topic of concern and research due to the probable health risks associated with aluminium exposure.
Studies have shown conflicting results, and regulatory bodies such as the U.S. Food and Drug Administration (FDA) and the European Chemicals Agency (ECHA) have established safety limits for using aluminium zirconium tetrachlorohydrex gly in consumer products.
However, more research is needed to understand the potential health impacts fully, and responsible use and adherence to regulations are crucial to ensure safety.
Sie effect of Aluminum Zirconium Tetrachlorohydrex Gly
One of the side effects is skin irritation or itching, which may occur due to the presence of chemicals in the antiperspirant that can irritate the skin.
In some cases, users may also experience redness, rash, or burning sensations on the skin. It is recommended to discontinue use and consult a doctor if any adverse reactions occur.
Alternatives and Future Trends
In recent years, there has been a growing demand for alternatives to AZT gly in consumer products, driven by increasing consumer awareness and preferences for natural or aluminium-free options.
Some alternatives include natural mineral salts, such as potassium alum or ammonium alum, and various plant-based ingredients, such as witch hazel, tea tree oil, and baking soda.
Additionally, there is ongoing research and development in the field of antiperspirants and deodorants,
to develop innovative formulations that provide effective odour and sweat control without using aluminium-based compounds.
Some emerging trends in this field include probiotics, enzymes, and nanotechnology to enhance the performance of antiperspirant and deodorant products.
Drugs Interaction of Aluminum Zirconium Tetrachlorohydrex Gly
Drug interactions have not been reported for this However, it is important to note that using certain antiperspirants or deodorants together with other topical medications may affect how well the other medications work. It is best to consult a healthcare provider before using aluminium zirconium tetrachlorohydrex gly together with any other medication or treatment.
Conclusion
It is a complex compound with wide-ranging applications in various industries, particularly antiperspirants, and deodorants.
Its chemical properties, synthesis, and manufacturing process have been elucidated, and its safety and regulatory considerations have been discussed.
As consumer preferences shift towards natural and aluminium-free options, there is a growing demand for alternatives and ongoing research in this field.
Responsible use, adherence to regulations, and continued research and development are essential to ensure the safe and effective use of AZT gly in various applications.
FAQs of Aluminum Zirconium Tetrachlorohydrex Gly
Q: What is aluminium zirconium tetrachlorohydrex gly?
Its an antiperspirant ingredient that reduces sweat and prevents body odor.
Q: How does aluminium zirconium tetrachlorohydrex gly work?
Its works by blocking the sweat ducts in the skin, preventing sweat from reaching the skin’s surface
Q: Is aluminum zirconium tetrachlorohydrex gly safe to use?
Yes, this drug is generally considered safe for use in antiperspirants.
Q: Are there any side effects of using antiperspirants containing AZT gly?
: Some people may face skin irritation or allergic reactions when using antiperspirants containing aluminium zirconium tetrachlorohydrex gly.
Q: Can aluminium zirconium tetrachlorohydrex gly cause breast cancer?
: There is no clear evidence to suggest that AZT gly or other antiperspirant ingredients are connected to an increased risk of breast cancer.
Q: Can aluminium zirconium tetrachlorohydrex gly stain clothing?
: Yes, antiperspirants containing aluminium zirconium tetrachlorohydrex gly may cause yellow staining on clothing over time.
Q: How often should I apply an antiperspirant containing aluminium zirconium tetrachlorohydrex gly?
: It is recommended to apply antiperspirants containing aluminium zirconium tetrachlorohydrex gly once a day, preferably in the evening before bed.
Q: Can I use antiperspirants containing aluminum zirconium tetrachlorohydrex gly if I have sensitive skin?
Anyone with sensitive skin may experience skin irritation or allergic reactions when using antiperspirants containing aluminum zirconium tetrachlorohydrex gly. Testing the product on a small skin area before applying it to a larger size is best.
.





